
Radioactive Iodine-131 Therapy:
From Pre-Diagnosis to Follow-up

Created By: Erin Grady, MD
As presented at the ACNM-SNM Conjoint Mid-Winter Meeting 2011
Overview
It should be noted that this module is only part of the curriculum. For more information about the full curriculum or have any questions, please email me.
This module should not be used as the sole source of information or as a replacement for a textbook. As with each topic you study, reading broadly is always recommended.
Therapy for non-differentiated thyroid cancers is not discussed in this module.
History of I-131 Therapy
Dr. Robert Henkin has said on multiple occasions, "You can't know where you're going until you know where you came from." Therefore, we will briefly visit the history of RAI therapy.
After the discovery of thyroid physiology and radioactive iodine, the way differentiated thyroid cancers are treated has forever been changed.
1938 was a big year for nuclear medicine, John Livingood & Glenn Seaborg discovered Iodine-131. 1941 marked the first therapeutic dose of I-131 by Saul Hertz. In 1946 the "atomic cocktail" of I-131 therapy was given to a patient with thyroid cancer, later approved by the FDA in 1951. You may have read that the thyroid has aided in the development of nuclear medicine. Indeed it has. A chapter in the book entitled Thyroid Cancer accounts this information. Additionally, I-131 was later added to everything from brain to renal agents for imaging and/or quantification.
Click here for more on the history of nuclear medicine.
Thyroid cancer accounts for 26,000 new cancer diagnoses in 2005 compared to a new cancer rate of > 250,000 in the United States. Despite this, thyroid cancer ranks as the 8th most common cancer in women. It has also been discussed to have the most rapidly rising incidence, though it is unclear if better detection methods (i.e. higher resolution of ultrasound) may be at least part of the cause. Fortunately, thyroid cancer has a very high 5-year survival rate of 97%. In a few slides, we'll discuss what factors can confer a worse prognosis.

Thyroid cancer happens to be the most common endocrine tumor in children. Though the histology is the same, the behavior of thyroid cancer in children is very different than that of adults as will be discussed later in this module.
Physical Characteristics of I-131
I-131:
tphys: 8.04 days
Principle gamma emmission: 364 keV
ß- emission as below
radiation dose: 1 rad/uCi (270mGy/MBq)
Iodine is taken up into differentiated thyroid cells via the Na-I symporter, an active transport process. Given this process, I-131 is an elegant way of treating differentiated thyroid cancers. As you recall from your basic physics, ß- deposits its energy in a very small distance, i.e. um-mm: this along with the long physical (and effective) half life aid in its favorable properties for a therapeutic radiopharmaceutical. Below is a very simplified decay scheme for I-131.
Simplified decay scheme for I-131:

I-123:
Cyclotron produced radionuclide.
tphys: 13.3 hours
Principle gamma emmission: 159 keV
Decays via electron capture. No ß- emission.
Crosses placenta & secreted in breastmilk
Tc-99m:
tphys: 6.02 hours
Principle gamma emmission: 140 keV
Decays via isomeric transition. No ß- emission.
Crosses placenta & secreted in breastmilk
Is not treated the exact same way as iodine by the thyroid gland: it is trapped but not organified.
----------------------------------------------------------------------------------
I-124:
tphys: 4.18 days
ß+ emission: 1.5 MeV, 23% yield (PET agent)
I-120:
tphys: 1.35 hours
ß+ emission: 4.6 MeV, 46% yield (PET agent)
I-122:
tphys: 3.6 min
ß+ emission: 3.1 MeV, 77% yield (PET agent)
Review of Thyroid Anatomy, Embryology & Histology
This is not meant to replace a thorough knowledge of anatomy, but is meant to hit some of the "high points" in development as well as gross and microscopic anatomy of the thyroid gland. This information will, in turn help you to remember some of the anatomic variations that are sometimes encountered.
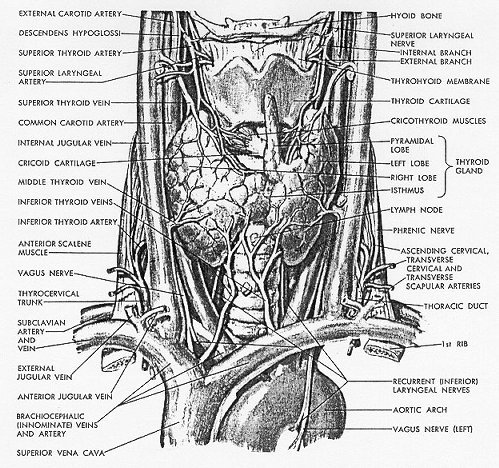
Embryology from e-medicine:
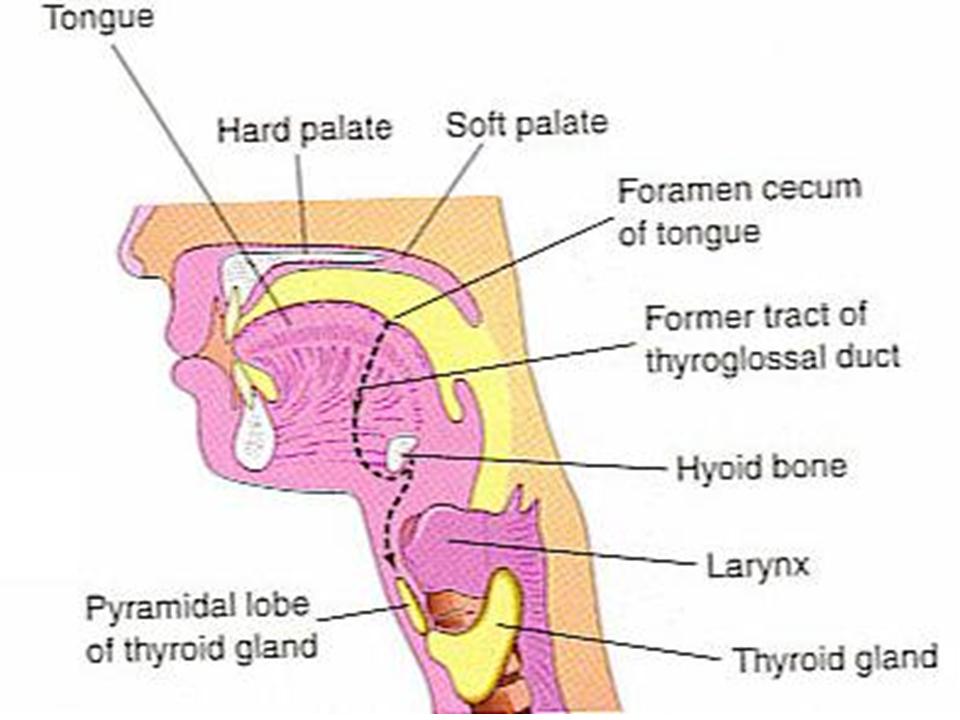
The thyroid gland is the first of the body's endocrine glands to develop, on approximately the 24th day of gestation. The gland originates as a proliferation of endodermal epithelial cells on the median surface of the developing pharyngeal floor. The site of this initial development lies between 2 key structures, the tuberculum impar and the copula, and is known as the foramen cecum. The thyroid initially develops caudal to the tuberculum impar, which is also known as the median tongue bud. This embryonic swelling arises from the first pharyngeal arch and occurs midline on the floor of the developing pharynx, eventually helping form the tongue as the 2 lateral lingual swellings overgrow it.
The foramen cecum begins rostral to the copula, also known as the hypobranchial eminence. This median embryologic swelling consists of mesoderm that arises from the second pharyngeal pouch (although the third and fourth pouches are also involved). The thyroid gland, therefore, originates from between the first and second pouches.
The initial thyroid precursor, the thyroid primordium, starts as a simple midline thickening and develops to form the thyroid diverticulum. This structure is initially hollow, although it later solidifies and becomes bilobed. The 2 lobes are located on either side of the midline and are connected via an isthmus.
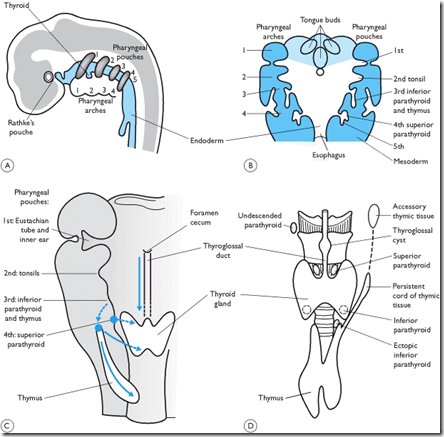
Some embryology-related abnormalities can cause diagnostic problems sometimes:
1) Thyroglossal fistula (the duct can persist)
2) Thyroglossal cyst (part of the duct persists)
3) Ectopic thyroid (tissue present along the duct)
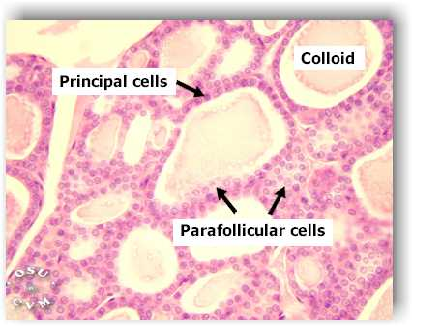
Normal thyroid histology:
Graves' Disease

Autonomously Functioning Nodule
MNG Histology/Thyroiditis

Papillary Thyroid Carcinoma

Follicular Thyroid Carcinoma

Why Iodine?
As described just a few pages ago:
Iodine is taken up into differentiated thyroid cells via the Na-I symporter, an active transport process. Given this process, I-131 is an elegant way of treating differentiated thyroid cancers. As you recall from your basic physics, ß- deposits its energy in a very small distance, i.e. um-mm. This along with the long physical (and effective) half life aid in its favorable properties for a therapeutic radiopharmaceutical. Below is a very simplified decay scheme for I-131.
Iodine taken orally is absorbed in the small bowel, trapped by the NaI symporter, oxodized and incorporated into T3, T4 and thyroglobulin.
Below is an image demonstrating the metabolism of Iodine in the human body. Keep these things in mind later on in the module when we are talking about some pitfalls of dosimetric imaging or false-positive scans.
The movement toward treating differentiated thyroid cancer was solidified after the observation that people had fewer recurrences following therapy with I-131 vs supression therapy with synthroid alone (El Mazzaferri & Endocrine Society).

Indications for RAI Therapy
 (s)
(s)

Therapy for Benign Conditions


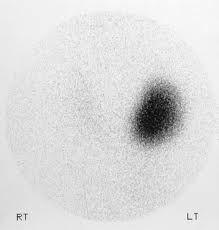

How Thyroid Uptake is Calculated

Measurements of the neck & thigh counts as well as the standard counts are taken at least twice and averaged.
Uptake can be calculated at 4 & 24 hours or 6 & 24 hours in cases of rapid iodine turnover (e.g. Grave's disease).

Interfering Medications & Conditions
It's true, these are numerous. This is also not an exhaustive list. Several articles have been writen on this subject, and they are listed below for your information. Additionally, a pharmicist may be able to help you regarding a medication's interference with RAIU and therapy.
Medications:
Adrenocorticosteroids
Amiodarone (most of this is iodine by weight)
Betadine
Bromides
Butazolidine
Cough medications
Iodine (SSKI, iodinated contrast material)
Kelp
Mercurials
Methimazole
Nitrates
Perchlorate
Propylthiouracil (PTU)
Salicylates (e.g. aspirin in large doses)
Sulfonamides
Sushi
Thiocynate
Vitamins
Conditions Causing Increased uptake:
Enzyme defects
Iodine deficiency or starvation
Hypoalbuminemia
Pregnancy
Tumor secretion of TSH
Conditions Causing Decreased Uptake:
Iodine overload
Renal failure
Thyroiditis
Tumors secreting thyroid hormone
Per the SNM procedure guidelines:

Large meals can slow absorption of ingested radioiodine and may interfere with early uptake measurements; therefore, the patient should avoid meals for at least 2 hours before and 2 hours after the oral dose of radioiodine if an early uptake is planned.

Patient Instructions for I-131: < 33* mCi:
I-131 Uptake & Scan in the Setting of Differentiated Thyroid Cancer**
or
Therapy for Benign Conditions
Here is an example the patient instructions compiled from various sources, based mostly on the instructions that are given at Loyola University Medical Center. These may be adapted to use at your institution if you wish. In general these are based on basic principles of radiation safety & NRC regulations.
INSTRUCTIONS:
Please follow these instructions for the next 3 days.
There are 3 basic principles to keep in mind:
You should avoid prolonged physical contact. It is particularly important in regards to contact with children and pregnant women because the thyroid glands of children and the unborn are more sensitive to the effects of radiation than those of adults.
During the next 3 days, avoid kissing and intimate contact.
If you are planning to become pregnant, consult with your doctor who can advise you on how long you should wait after treatment. We typically advise not becoming pregnant during the year following a dose of I-131.
If you have a baby or you are taking care of one, you can probably do all the things necessary to care for your baby, except breastfeeding. It is preferable not to keep the baby too close to your body, such as sitting in your lap for more than a short time during the next 3 days.
If you have been breastfeeding your baby, you must stop. Radioiodine passes into breast milk and may cause unwanted effects in the nursing baby, such as an underactive thyroid. This can have serious impact on the developing baby. You will not be able to resume breastfeeding.
Wash your hands thoroughly with soap and plenty of water each time after you go to the toilet, alcohol gel will not remove the iodine. Keep the toilet especially clean. Be sure to flush it 2 or 3 times with the lid down***.
Rinse the bathroom sink and tub thoroughly after you use them. Clean bathroom habits will reduce the chances of others becoming contaminated by the radioiodine.
No one else should be able to accidentally use your personal effects (toothbrush, washcloth, etc) since iodine might be spread that way. Additionally, you may choose to launder your soiled clothes and linens separately.
Drink plenty of liquids such as water or juices. This will make you urinate more frequently and help the radioiodine to leave your body more rapidly, thus lowering the amount of radioiodine in your body.
Finally, if you should have any questions, you should feel free to contact any of the physicians in the Section of Nuclear Medicine. During the weekdays during business hours, you can call this number. After hours, call the hospital operator and ask them to page the Nuclear Medicine physician on call.
These guidelines will limit exposure to others far below acceptable levels. Radioactive iodine will disappear completely through your own body excretions.
*Some agreement states (states that have rules stricter than NRC) will use 30 mCi as the cut off, check with your state.
**For patients receiving I-123 uptake & scan no precautions are generally given (other than breastfeeding cautions) since the gamma-ray energy is less and there is less risk to another individual's thyroid gland (no B-).
*** Some controversy exists about whether or not to flush the toilet twice. If your patient is sharing a bathroom, this may be especially important as there is a trap under the toilet which can hold radioactive waste after one flush prior to a second flush which can move that waste out of the area adjacent to the toilet. This would minimize exposure to someone else using the toilet. It is recommended that a patient use their own bathroom if possible


Factors Affecting Risk of Differentiated Thyroid Carcinomas
In Adults:
In Children:

In case you'd like to know a bit more on the molecular level...here are some of the:

Thyroid Cancer Staging

http://www.cancerstaging.org/index.html

Deciding Inpatient or Outpatient for I-131 Therapy > 33 mCi
Therapy for Differentiated Thyroid Cancer
In 1997, the NRC changed the rules to allow some qualified patients to be quarantined at home after receiving their I-131 therapy. There are a number of things that must be taken into consideration. The following is excerpted from the ATA Guidelines for I-131 Therapy and aids in deciding who is appropriate for outpatient therapy. For more discussion on this topic, please see the controversial section (topic 5).

If the patient has a family member who is pregnant or young children or they are the primary caregiver for someone, we usually recommend treating the patient as an inpatient to ensure that their I-131 is not spread to others in their household. For more on this, see the controversial section (topic 5).

Patient Instructions for Outpatient I-131: > 33 mCi:
Therapy in the Setting of Differentiated Thyroid Cancer
There is significant controversy about the proper guidelines to be given to a patient with regard to I-131 therapy. The only thing that is not controversial is that they should be in accordance with the NRC guidelines and involve stricter instructions to patients than the < 33 mCi therapies, in general the patient will be in isolation until determined by your radiation safety measurements (< 7 millirem/hour at 1 meter) and calculations (patient-specific calculation indicates that potential TEDE to any individual would not be greater than 500 millirem).
There are a preponderance of instructions, they vary in strictness and it should be noted that several studies of the current practice have reported that radiation exposure to household members of patients receiving outpatient radioiodine therapy for hyperthyroidism or thyroid carconima were almost always well below the limit and some neared the limit. Once again, patients should not stay in hotels or use public transportation to and from their therapy--if they cannot, an inpatient therapy would be better.
There is no question that instructions must be given to patients and is best done by the physician or RSO and preferably not the technologist (I love our technologists, but this is one less thing they should have to do).
Resources include: SNM Procedure guidelines for I-131 therapy, Thyroid Cancer (textbook), ATA Guidelines. It should be noted that the ATA Guidelines have not been approved by the Society of Nuclear Medicine and are very restrictive; there has been significant controversy over these guidelines in particular. It should be noted that none of the guidelines are "evidence based", but all of them do conform to the NRC requirements for decreasing exposure to the public, especially pregnant women and children who are the most radiosensitive. As always we should remember the ALARA (as low as reasonably achievable) principle.

Common Patient Questions
The general public in general has great fear of radioactivity...and also admiration (e.g. Spiderman). In this section, we will discuss some of the common patient questions and their answers.
In discussing patient questions prior to an RAI therapy, an effort to exercise patience and compassion should always be made. These questions are important to the patient so do take the time you spend with them seriously.

|
Patient question |
Potential answer |
|
Will this dose cause me to develop cancer later? |
This has been extensively examined and most studies do not show an increase in secondary malignancies--so the answer from most studies is NO. Some have however suggested an increase in leukemia, colorectal, bladder, or breast cancer but only slightly greater than the general population risk. The risk of not undergoing the I-131 therapy may outweigh the risk of possible second primary depending on the patient's risk for disease. This should be carefully considered between the treating physician and the patient's endocrinologist and discussed with the patient.1 |
|
Is my dog or cat going to be ok?
|
Overall, the answer is yes as long as they don't drink out of the toilet. Some people advise that the dog or cat should not sleep in the bed with you as they may inadvertently take some activity from you around the house. Cats are particularly sensitive to I-131, so make sure they do not come into contact with any excreted products; if they are physically present near you it will not harm them.
|
|
Will this stuff that I take contaminate my whole house?
|
No and yes...depending on the hygiene practices used. As in the previous page, good hand hygiene, refraining from: use of the gym (sweating), intimacy, sharing of bed/towels/toothbrush and bathroom greatly reduce the risk of contaminating the whole house. Some websites seem to advocate for more precautions, but in general that may be slightly overcautious. Following the given instructions should be sufficient. |
|
Will I glow in the dark or get superpowers?
|
Unfortunately no and no. Sorry...that would be fun.
|
|
When can I get pregnant again? What about breastfeeding? |
The length of time after therapy before attempting to become pregnant is recommended at ~ 6 months to 1 year. It is strongly discouraged that you become pregnant during the time following your RAI therapy. This is because the thyroid hormones need to be well-regulated for pregnancy; there has been no significant increase in congential agnormalities in babies born from mothers with prior I-131 therapy. Breastfeeding after an RAI therapy should be completely discontinued for your current child you are breastfeeding. |
|
Will the RAI therapy affect my menstral periods? |
It is possible that your period might stop for a month or two. This is called "transient ovarian failure" usually seen in higher therapy doses.
|
|
If I'm an inpatient, what sorts of things should I expect? |
In general, you will be in an isolated room and released when your radiation level falls below a certain point determined by the government. There are strict time restrictions on visitors (no more than a few minutes). Belongings you bring with you will be checked for radioiodine before you leave, if they are contaminated, they will not be given back to you immediately--they would be kept for about 10 weeks, so don't bring something you can't be without for a while. Nurses will provide as needed. Housekeeping will be limited in your room, the radiation safety team will be the ones taking care of anything contaminated. Flush your toilet with the lid shut.
|
|
If I'm having my RAI therapy for hyperthyroidism, will that cause me to develop thyroid cancer later? |
While it is true that radiation is one of the known risk factors for development of thyroid cancer, therapy with I-131 for hyperthyroidism has not shown a statistically significant increase in thyroid cancer.1 |
1. Hoffman DA, McConahey WM, Diamond EL, Kurland LT. Cancer incidence following treatment of hyperthyroidism. Int J Epidemiol. 1982. 11:218-224.
Holm L-E, Dahlqvist I, Israelsson A, Lundell G. Malignant thyroid tumors after iodine-131 therapy: a retrospective cohort study. N Engl J Med. 1980; 303:188-191.

Possible Side Effects
RAIU with I-123
RAIU with I-131

RAI Therapy (I-131) for Hyperthyroidism
RAI Therapy (I-131) for Differentiated Thyroid Carcinomas
These things should be mentioned during the consent process for patients undergoing therapy as well as things to help prevent side effects (i.e. drinking copious fluids, sour candies, etc.)

Good Patient Resources
Commonly, patients may come to you asking about resources from things to what they can eat on a low-iodine diet to what they can expect during and following the RAI therapy.
Below are some links that are useful in answering patient's questions, particularly those that you may not have a quick answer to.

http://www.cancer.gov/cancertopics/types/thyroid
http://www.thyroid.org/patients/links.html
http://www.lightoflifefoundation.org/Resources/Low-Iodine-Cookbook
http://www.cancercare.org/get_help/help_by_diagnosis/diagnosis.php?diagnosis=thyroid
Release criteria & the "Get Out of Jail Free Card"

What we refer to a "get out of jail free card" is a small slip of paper indicating that the patient has been treated with RAI therapy or dosimetric scan performed with I-131. Radiation detection is performed in some cases at state lines and also at airports. It is important for the patient to understand that they may be pulled aside if they are traveling. The "get out of jail free card" should also indicate a telephone number of your department in case they are pulled aside. In some cases, a business card is given for this purpose.
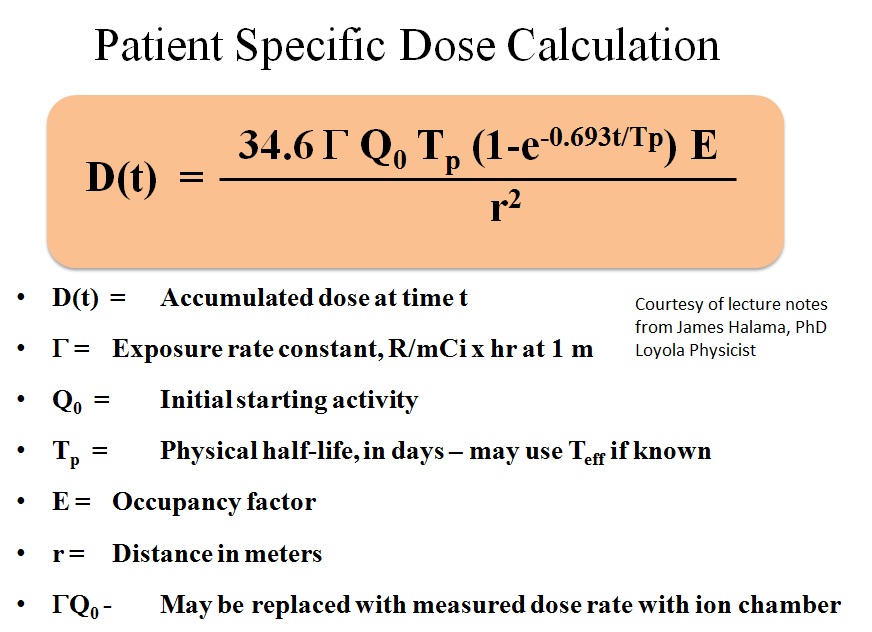
As set forth by the NRC, there are specific release criteria, it is important to know the NRC regulations as well as any possible more restrictive criteria specified by the state you practice in. Asking your radiation safety department is a good place to start as they are usually intimately involved with assessing the patients for release. Below is the formula used for calculating dose to a patient as well as an example problem. These formulas can be put into an excel file to facilitate these calculations as they are done often.
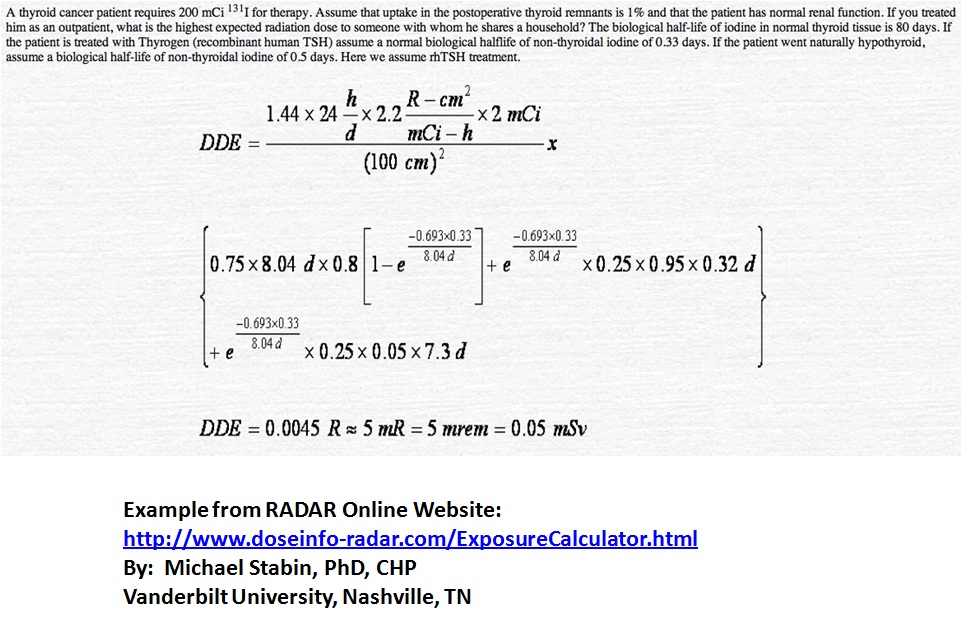
Other nice radiation dose resources are located at the following website:
http://www.doseinfo-radar.com/

What is a Written Directive?

A written directive is akin to a prescription for I-131 therapy and must contain the following items per the NRC Regulations and must be signed by an authorized user or someone with the appropriate credentials to prescribe I-131.
§ 35.40 Written directives.
(a) A written directive must be dated and signed by an authorized user before the administration of I-131 sodium iodide greater than 1.11 megabecquerels (MBq) (30 microcuries (µCi)), any therapeutic dosage of unsealed byproduct material or any therapeutic dose of radiation from byproduct material.
(1) If, because of the emergent nature of the patient's condition, a delay in order to provide a written directive would jeopardize the patient's health, an oral directive is acceptable. The information contained in the oral directive must be documented as soon as possible in writing in the patient's record. A written directive must be prepared within 48 hours of the oral directive.
(b) The written directive must contain the patient or human research subject's name and the following information--
(1) For any administration of quantities greater than 1.11 MBq (30 µCi) of sodium iodide I-131: the dosage;
(2) For an administration of a therapeutic dosage of unsealed byproduct material other than sodium iodide I-131: the radioactive drug, dosage, and route of administration;
(3) For gamma stereotactic radiosurgery: the total dose, treatment site, and values for the target coordinate settings per treatment for each anatomically distinct treatment site;
(4) For teletherapy: the total dose, dose per fraction, number of fractions, and treatment site;
(5) For high dose-rate remote afterloading brachytherapy: the radionuclide, treatment site, dose per fraction, number of fractions, and total dose; or
(6) For all other brachytherapy, including low, medium, and pulsed dose rate remote afterloaders:
(i) Before implantation: treatment site, the radionuclide, and dose; and
(ii) After implantation but before completion of the procedure: the radionuclide, treatment site, number of sources, and total source strength and exposure time (or the total dose).
(c) A written revision to an existing written directive may be made if the revision is dated and signed by an authorized user before the administration of the dosage of unsealed byproduct material, the brachytherapy dose, the gamma stereotactic radiosurgery dose, the teletherapy dose, or the next fractional dose.
(1) If, because of the patient's condition, a delay in order to provide a written revision to an existing written directive would jeopardize the patient's health, an oral revision to an existing written directive is acceptable. The oral revision must be documented as soon as possible in the patient's record. A revised written directive must be signed by the authorized user within 48 hours of the oral revision.
(d) The licensee shall retain a copy of the written directive in accordance with § 35.2040.
[67 FR 20370, Apr. 24, 2002 as amended at 68 FR 75389, Dec. 31, 2003]
Review of the Pertinent NRC Regulations
Having a very good understanding of these regulations is impative in dealing with therapy and in general for handling radiopharmaceuticals. Below you will find a few important and pertinent sections excerpted.
10 CRF, Part 35 deals with medical use of radiopharmaceuticals.
Subpart E--Unsealed Byproduct Material--Written Directive Required
In addition to the requirements of § 19.12 of this chapter,
(a) A licensee shall provide radiation safety instruction, initially and at least annually, to personnel caring for patients or human research subjects who cannot be released under § 35.75. To satisfy this requirement, the instruction must be commensurate with the duties of the personnel and include—
(1) Patient or human research subject control;
(2) Visitor control, including—
(i) Routine visitation to hospitalized individuals in accordance with § 20.1301(a)(1) of this chapter; and
(ii) Visitation authorized in accordance with § 20.1301(c) of this chapter;
(3) Contamination control;
(4) Waste control; and
(5) Notification of the Radiation Safety Officer, or his or her designee, and an authorized user if the patient or the human research subject has a medical emergency or dies.
(b) A licensee shall retain a record of individuals receiving instruction in accordance with § 35.2310.
[67 FR 20370, Apr. 24, 2002, as amended at 68 FR 19324, Apr. 21, 2003]
Except as provided in § 35.57, the licensee shall require an authorized user for the oral administration of sodium iodide I-131 requiring a written directive in quantities less than or equal to 1.22 Gigabecquerels (33 millicuries), to be a physician who—
(a) Is certified by a medical specialty board whose certification process includes all of the requirements in paragraphs (c)(1) and (c)(2) of this section and whose certification process has been recognized by the Commission or an Agreement State and who meets the requirements in paragraph (c)(3) of this section. (The names of board certifications which have been recognized by the Commission or an Agreement State will be posted on the NRC's Web page.); or
(b) Is an authorized user under § 35.390 for uses listed in § 35.390(b)(1)(ii)(G)(1) or (2), § 35.394, or equivalent Agreement State requirements; or
(c)(1) Has successfully completed 80 hours of classroom and laboratory training, applicable to the medical use of sodium iodide I-131 for procedures requiring a written directive. The training must include—
(i) Radiation physics and instrumentation;
(ii) Radiation protection;
(iii) Mathematics pertaining to the use and measurement of radioactivity;
(iv) Chemistry of byproduct material for medical use; and
(v) Radiation biology; and
(2) Has work experience, under the supervision of an authorized user who meets the requirements in §§ 35.57, 35.390, 35.392, 35.394, or equivalent Agreement State requirements. A supervising authorized user who meets the requirements in § 35.390(b) must also have experience in administering dosages as specified in §§ 35.390(b)(1)(ii)(G)(1) or 35.390(b)(1)(ii)(G)(2). The work experience must involve—
(i) Ordering, receiving, and unpacking radioactive materials safely and performing the related radiation surveys;
(ii) Performing quality control procedures on instruments used to determine the activity of dosages and performing checks for proper operation of survey meters;
(iii) Calculating, measuring, and safely preparing patient or human research subject dosages;
(iv) Using administrative controls to prevent a medical event involving the use of byproduct material;
(v) Using procedures to contain spilled byproduct material safely and using proper decontamination procedures; and
(vi) Administering dosages to patients or human research subjects, that includes at least 3 cases involving the oral administration of less than or equal to 1.22 gigabecquerels (33 millicuries) of sodium iodide I-131; and
(3) Has obtained written attestation that the individual has satisfactorily completed the requirements in paragraphs (c)(1) and (c)(2) of this section, and has achieved a level of competency sufficient to function independently as an authorized user for medical uses authorized under § 35.300. The written attestation must be signed by a preceptor authorized user who meets the requirements in §§ 35.57, 35.390, 35.392, 35.394, or equivalent Agreement State requirements. A preceptor authorized user, who meets the requirement in § 35.390(b), must also have experience in administering dosages as specified in §§ 35.390(b)(1)(ii)(G)(1) or 35.390(b)(1)(ii)(G)(2).
[67 FR 20370, Apr. 24, 2002, as amended at 68 FR 19325, Apr. 21, 2003; 68 FR 75389, Dec. 31, 2003; 69 FR 55738, Sep. 16, 2004; 70 FR 16364, Mar. 30, 2005; 71 FR 15009, Mar. 27, 2006; 74 FR 33905, Jul. 14, 2009]
Except as provided in 35.57, the licensee shall require an authorized user for the oral administration of sodium iodide I-131 requiring a written directive in quantities greater than 1.22 Gigabecquerels (33 millicuries), to be a physician who—
(a) Is certified by a medical specialty board whose certification process includes all of the requirements in paragraphs (c)(1) and (c)(2) of this section, and whose certification has been recognized by the Commission or an Agreement State, and who meets the requirements in paragraph (c)(3) of this section. (The names of board certifications which have been recognized by the Commission or an Agreement State will be posted on the NRC's Web page.); or
(b) Is an authorized user under § 35.390 for uses listed in § 35.390(b)(1)(ii)(G)(2) or equivalent Agreement State requirements; or
(c)(1) Has successfully completed 80 hours of classroom and laboratory training, applicable to the medical use of sodium iodide I-131 for procedures requiring a written directive. The training must include—
(i) Radiation physics and instrumentation;
(ii) Radiation protection;
(iii) Mathematics pertaining to the use and measurement of radioactivity;
(iv) Chemistry of byproduct material for medical use; and
(v) Radiation biology; and
(2) Has work experience, under the supervision of an authorized user who meets the requirements in §§ 35.57, 35.390, 35.394, or equivalent Agreement State requirements. A supervising authorized user, who meets the requirements in § 35.390(b), must also have experience in administering dosages as specified in § 35.390(b)(1)(ii)(G)(2). The work experience must involve—
(i) Ordering, receiving, and unpacking radioactive materials safely and performing the related radiation surveys;
(ii) Performing quality control procedures on instruments used to determine the activity of dosages and performing checks for proper operation of survey meters;
(iii) Calculating, measuring, and safely preparing patient or human research subject dosages;
(iv) Using administrative controls to prevent a medical event involving the use of byproduct material;
(v) Using procedures to contain spilled byproduct material safely and using proper decontamination procedures; and
(vi) Administering dosages to patients or human research subjects, that includes at least 3 cases involving the oral administration of greater than 1.22 gigabecquerels (33 millicuries) of sodium iodide I-131; and
(3) Has obtained written attestation that the individual has satisfactorily completed the requirements in paragraphs (c)(1) and (c)(2) of this section, and has achieved a level of competency sufficient to function independently as an authorized user for medical uses authorized under § 35.300. The written attestation must be signed by a preceptor authorized user who meets the requirements in §§ 35.57, 35.390, 35.394, or equivalent Agreement State requirements. A preceptor authorized user, who meets the requirements in § 35.390(b), must also have experience in administering dosages as specified in § 35.390(b)(1)(ii)(G)(2).
[67 FR 20370, Apr. 24, 2002, as amended at 68 FR 19325, Apr. 21, 2003; 68 FR 75389, Dec. 31, 2003; 69 FR 55739, Sep. 16, 2004; 70 FR 16365, Mar. 30, 2005; 71 FR 15010. Mar. 27, 2006; 74 FR 33905, Jul. 14, 2009]
(a) A licensee shall report any event, except for an event that results from patient intervention, in which the administration of byproduct material or radiation from byproduct material results in--
(1) A dose that differs from the prescribed dose or dose that would have resulted from the prescribed dosage by more than 0.05 Sv (5 rem) effective dose equivalent, 0.5 Sv (50 rem) to an organ or tissue, or 0.5 Sv (50 rem) shallow dose equivalent to the skin; and
(i) The total dose delivered differs from the prescribed dose by 20 percent or more;
(ii) The total dosage delivered differs from the prescribed dosage by 20 percent or more or falls outside the prescribed dosage range; or
(iii) The fractionated dose delivered differs from the prescribed dose, for a single fraction, by 50 percent or more.
(2) A dose that exceeds 0.05 Sv (5 rem) effective dose equivalent, 0.5 Sv (50 rem) to an organ or tissue, or 0.5 Sv (50 rem) shallow dose equivalent to the skin from any of the following--
(i) An administration of a wrong radioactive drug containing byproduct material;
(ii) An administration of a radioactive drug containing byproduct material by the wrong route of administration;
(iii) An administration of a dose or dosage to the wrong individual or human research subject;
(iv) An administration of a dose or dosage delivered by the wrong mode of treatment; or
(v) A leaking sealed source.
(3) A dose to the skin or an organ or tissue other than the treatment site that exceeds by 0.5 Sv (50 rem) to an organ or tissue and 50 percent or more of the dose expected from the administration defined in the written directive (excluding, for permanent implants, seeds that were implanted in the correct site but migrated outside the treatment site).
(b) A licensee shall report any event resulting from intervention of a patient or human research subject in which the administration of byproduct material or radiation from byproduct material results or will result in unintended permanent functional damage to an organ or a physiological system, as determined by a physician.
(c) The licensee shall notify by telephone the NRC Operations Center3 no later than the next calendar day after discovery of the medical event.
(d) By an appropriate method listed in § 30.6(a) of this chapter, the licensee shall submit a written report to the appropriate NRC Regional Office listed in § 30.6 of this chapter within 15 days after discovery of the medical event.
(1) The written report must include--
(i) The licensee's name;
(ii) The name of the prescribing physician;
(iii) A brief description of the event;
(iv) Why the event occurred;
(v) The effect, if any, on the individual(s) who received the administration;
(vi) What actions, if any, have been taken or are planned to prevent recurrence; and
(vii) Certification that the licensee notified the individual (or the individual's responsible relative or guardian), and if not, why not.
(2) The report may not contain the individual's name or any other information that could lead to identification of the individual.
(e) The licensee shall provide notification of the event to the referring physician and also notify the individual who is the subject of the medical event no later than 24 hours after its discovery, unless the referring physician personally informs the licensee either that he or she will inform the individual or that, based on medical judgment, telling the individual would be harmful. The licensee is not required to notify the individual without first consulting the referring physician. If the referring physician or the affected individual cannot be reached within 24 hours, the licensee shall notify the individual as soon as possible thereafter. The licensee may not delay any appropriate medical care for the individual, including any necessary remedial care as a result of the medical event, because of any delay in notification. To meet the requirements of this paragraph, the notification of the individual who is the subject of the medical event may be made instead to that individual's responsible relative or guardian. If a verbal notification is made, the licensee shall inform the individual, or appropriate responsible relative or guardian, that a written description of the event can be obtained from the licensee upon request. The licensee shall provide such a written description if requested.
(f) Aside from the notification requirement, nothing in this section affects any rights or duties of licensees and physicians in relation to each other, to individuals affected by the medical event, or to that individual's responsible relatives or guardians.
(g) A licensee shall:
(1) Annotate a copy of the report provided to the NRC with the:
(i) Name of the individual who is the subject of the event; and
(ii) Social security number or other identification number, if one has been assigned, of the individual who is the subject of the event; and
(2) Provide a copy of the annotated report to the referring physician, if other than the licensee, no later than 15 days after the discovery of the event.
[68 FR 58805, Oct. 10, 2003]
3 The commercial telephone number of the NRC Operations Center is (301) 951-0550.
http://www.nrc.gov/reading-rm/doc-collections/cfr/

Importance of a RECENT Pregnancy Test
A patient must have a pregnancy test within 72 hours, but on the day of the therapy or uptake/scan with I-131 is better. Depending on timing after conception, a standard pregnancy test does have the potential to be falsely negative.
Should a patient let you know that she was pregnant at the time of the dosing, dosimetry to the fetus should be performed. In general, the effects this early in pregnancy are all or none; i.e. result in spontaneous abortion or the fetus will progress normally to birth.
If a patient who is pregnant at the 10-12 week of gestation or later receives I-131, there is potential for iatrogenic hypothyroidism and the associated mental side-effects. If you wish to further review embryology of the thyroid, you can review page 5 (click on 5 at the top of the page).
Regardless of gestational age, the NRC must be notified. The regulations are excerpted and are below:
(a) A licensee shall report any dose to an embryo/fetus that is greater than 50 mSv (5 rem) dose equivalent that is a result of an administration of byproduct material or radiation from byproduct material to a pregnant individual unless the dose to the embryo/fetus was specifically approved, in advance, by the authorized user.
(b) A licensee shall report any dose to a nursing child that is a result of an administration of byproduct material to a breast-feeding individual that--
(1) Is greater than 50 mSv (5 rem) total effective dose equivalent; or
(2) Has resulted in unintended permanent functional damage to an organ or a physiological system of the child, as determined by a physician.
(c) The licensee shall notify by telephone the NRC Operations Center no later than the next calendar day after discovery of a dose to the embryo/fetus or nursing child that requires a report in paragraphs (a) or (b) in this section.
(d) By an appropriate method listed in § 30.6(a) of this chapter, the licensee shall submit a written report to the appropriate NRC Regional Office listed in § 30.6 of this chapter within 15 days after discovery of a dose to the embryo/fetus or nursing child that requires a report in paragraphs (a) or (b) in this section.
(1) The written report must include--
(i) The licensee's name;
(ii) The name of the prescribing physician;
(iii) A brief description of the event;
(iv) Why the event occurred;
(v) The effect, if any, on the embryo/fetus or the nursing child;
(vi) What actions, if any, have been taken or are planned to prevent recurrence; and
(vii) Certification that the licensee notified the pregnant individual or mother (or the mother's or child's responsible relative or guardian), and if not, why not.
(2) The report must not contain the individual's or child's name or any other information that could lead to identification of the individual or child.
(e) The licensee shall provide notification of the event to the referring physician and also notify the pregnant individual or mother, both hereafter referred to as the mother, no later than 24 hours after discovery of an event that would require reporting under paragraph (a) or (b) of this section, unless the referring physician personally informs the licensee either that he or she will inform the mother or that, based on medical judgment, telling the mother would be harmful. The licensee is not required to notify the mother without first consulting with the referring physician. If the referring physician or mother cannot be reached within 24 hours, the licensee shall make the appropriate notifications as soon as possible thereafter. The licensee may not delay any appropriate medical care for the embryo/fetus or for the nursing child, including any necessary remedial care as a result of the event, because of any delay in notification. To meet the requirements of this paragraph, the notification may be made to the mother's or child's responsible relative or guardian instead of the mother. If a verbal notification is made, the licensee shall inform the mother, or the mother's or child's responsible relative or guardian, that a written description of the event can be obtained from the licensee upon request. The licensee shall provide such a written description if requested.
(f) A licensee shall:
(1) Annotate a copy of the report provided to the NRC with the:
(i) Name of the pregnant individual or the nursing child who is the subject of the event; and
(ii) Social security number or other identification number, if one has been assigned, of the pregnant individual or the nursing child who is the subject of the event; and
(2) Provide a copy of the annotated report to the referring physician, if other than the licensee, no later than 15 days after the discovery of the event.
[68 FR 58805, Oct. 10, 2003]
A Word on Agreement States
An agreement state is a state that has agreed to make regulations and enforce their own reglations that are more restrictive than the NRC. There are a number of agreement states. They are listed below. If your state is an agreement state, make an effort to become familiar with the regulations put forth by your state.
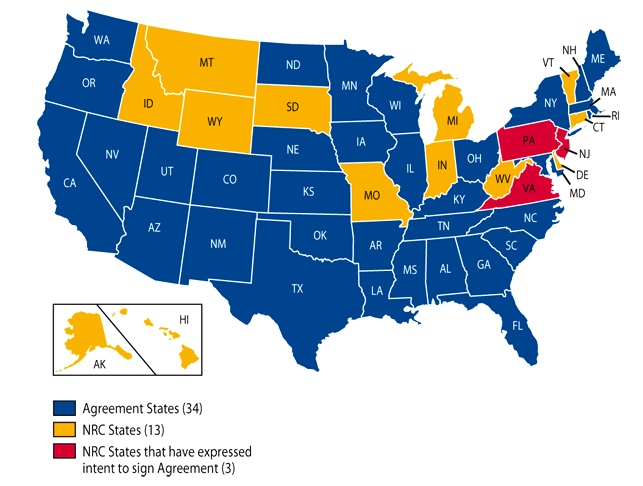

Lactation Status Considerations

Determining the Dose
Determining the dose can be controversial, but here are some of the currently accepted approaches.
Many would argue that the first time is the best time you will ever have to treat remaining thyroid cancer cells and not to "skimp" on the dose.

Following-up Thyroid Cancer
It is commonplace to receive an order a thyroid therapy for rising thyroglobulin levels. Care in approving this order is needed. The practice at Loyola is to always first do a dosimetric scan to see if there is evidence of uptake to suggest progression of disease. In the case that this is demonstrated, therapy is given. If there is no evidence of disease, a PET or PET/CT scan with F-18 FDG is acquired. If there is a hypermetabolic focus or foci, I-131 therapy is not given. This is because the thyroid cancer cells are no longer differentiated and will not be ablated by a high dose I-131 therapy. In these cases, you dose the patient with radiation for no clear benefit. Therefore, it is important to NOT treat thyroglobulin levels blindly.
Throglobulin is a 670 kDa glycopeptide and is the major product of thyroid follicular cells. The rate of production is influenced by elevated levels of TSH. Usually, only small amounts of thyroglobulin are in the circulation and are proportional to the thyroid mass. Reasons for increased thyroglobulin include: inflammation (thyroiditis), low iodine intake, cigarette smoking. Following total thyroidectomy for thyroid malignancy, the thyroglobulin level should be below the detectable limit.
Antithyroglobulin antibodies can also present a problem. They are commonly encountered autoantibodies in patients with or without thyroid cancer. The presence of high-titer antithyroglobulin antibodies suggests the presence of residual thyroid cancer. These antibodies can also cause erroneous thyroglobulin assay results so antithyroglobulin antibodies should be ordered when ordering a thyroglobulin assay.
You may also see results of a "Stimulated" thyroglobulin. This is done with withdrawl from thyroid hormone or synthetic TSH (rhTSH aka Thyrogen injections) and increases sensitivity for detecting any abnormal thyroglobulin levels. Non-stimulated thyroglobulin levels taken during TSH suppression are also important, and represent higher specificity since the TSH is low. As we know, it is important for patients with thyroid cancer to keep the TSH suppressed when we are not occasionally screening these labs or preparing for therapy so as not to stimulate any remaining thyroid cells.
Below is the reasoning behind FDG PET when Iodine uptake is not seen:
There are cases where some patients have received a significant radiation dose from an I-131 therapy to non-avid disease based only on what is seen from thyroglobulin levels. Completing the work-up with FDG PET is therefore important. Therapeutic management changes significantly in non-iodine avid disease and is outside the scope of this module.

Below are a few charts from the ATA regarding similar topics:
Thyroid. Volume 19, Number 11, 2009




A Few Controversial Topics
For each of the below controversial topics, they are discussed objectively as possible.

1) Stunning
2) rhTSH vs Thyroid hormone withdrawal

3) Dosimetry based therapeutic dose vs Empiric therapeutic dose
4) Use of Lithium augmentation
5) Inpatient vs. Outpatient High Dose Therapies
The Dosimetric Scan & A Few Pitfalls
Potential False Positives on RAI Whole-Body Scans
As described before, the iodine or iodide has many locations it can go in the body; the most useful for our purposes is the thyroid, however sometimes it can present a problem in diagnostic tests, so using your detective skills may come in handy. We revisit the locations of iodine metabolism.
Test Your Knowledge
Please email to ask any questions you may have and for a copy of the quiz; versions will change from time to time.
The quiz will include a physics problem on an actual previous patient with regard to dosimetry. After you have completed your test, you will get an email back detailing any missed questions and reasoning for correct answers.
Literature Cited, Interesting Papers & Helpful Resources